Department of Defense Program Funds Study of Cranial Regeneration
Biomedical engineering researchers at UConn Health believe there might be a way to use ultrasound to compel the body to regrow cranial tissue.
Yusuf Khan, an associate professor of orthopedic surgery, and Dr. David Hersh, associate professor of neurosurgery, have been studying whether some principles of bone development in children could apply to bone healing in adults who’ve had part of their skull removed and replaced.
They recently were awarded a two-year grant totaling $435,000 through the Congressionally Directed Medical Research Program’s Peer Reviewed Medical Research Program, part of the Department of Defense.
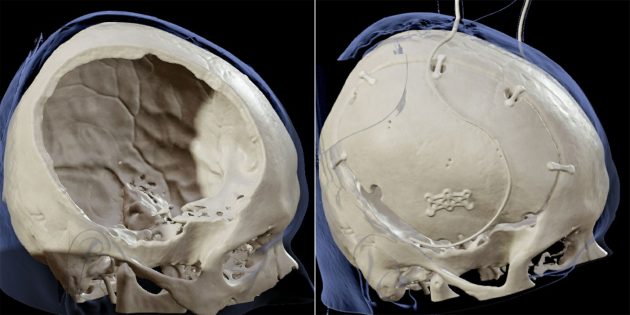
A decompressive craniectomy, or the removal of a portion of the skull, is a potentially life-saving intervention for when a patient suffers from brain edema, or severe swelling, such as when there has been a traumatic brain injury. The procedure gives the swelling brain more space, relieving pressure and lowering the risk of herniation, which can be fatal.
Hersh, a pediatric neurosurgeon at Connecticut Children’s who performs craniectomies on select patients with certain conditions, notes that after the follow-up cranioplasty, which is when the portion of skull that had been removed is then reattached, that piece of bone can have problems reintegrating with the remainder of the skull. In some cases, the bone gets resorbed, meaning it instead starts to shrink and get absorbed by the body.
“You end up being left with big gaps in the bone, which can leave the underlying brain at risk,” Hersh says. “And then the patient needs even more surgeries to provide appropriate coverage, which might involve a synthetic replacement.”

Original bone has many biological and other advantages over synthetic materials, such as metals or hard plastics, and trying to eliminate or reduce the need for synthetics is one of the tenets of regenerative engineering.
In 2019, Hersh started collaborating with Khan, who had been studying therapeutic ultrasound and how it facilitates fracture repair. Hersh had prior experience using therapeutic ultrasound for neurosurgical applications such as for blood brain barrier opening.
“David came to me with a very specific pediatric problem that he wanted to try to solve,” Khan says. “This grant really grew from the original pediatric application, but, through us working together over the years, we realized the potential for adults, too. And the Congressionally Directed Medical Research Program is an ideal funder for a project like this because of the type of battlefield injuries that soldiers unfortunately experience.”
The focus is on the dura, the thin layer of tissue that encloses the brain, and whether low-intensity ultrasound can provide a physical force that the cells can sense, possibly stimulating cranial bone regeneration.
“We think that there’s something unique about those dural cells in that they respond to physical forces, just like bone cells do,” Khan says. “We’ve seen interesting responses by dural cells from young animals that are exposed to ultrasound, and we’re now going to explore whether skeletally mature cells act the same way. We plan to add stem cells to the defect site to study how they communicate with dural cells and whether this can stimulate new bone formation.”

Khan likens it to how certain fractures actually benefit from weight-bearing during the healing process.
Hersh says the body already provides an encouraging clue.
“Our hypothesis is based on what people have learned about normal development –the skull grows in response to the underlying dura releasing signals that then stimulate bone formation,” Hersh says. “We think that happens as a result of the brain itself growing when we’re young and applying mechanical strain to the dura, which then signals to the bone above it. So, our aim is to recreate that natural process to facilitate bone healing in a way that’s similar to the original bone development.”
While studying this issue may have utility for wounded warriors, its potential applications may extend far beyond that. Examples include patients undergoing a decompressive craniectomy and subsequent cranioplasty for reasons unrelated to combat, including in the setting of civilian traumatic brain injury and certain severe types of stroke.
“This collaboration on regenerating cranial bone is so important for the future of our wounded warriors,” says Dr. Cato T. Laurencin, the founder and director of the Cato T. Laurencin Institute for Regenerative Engineering. “It is also beneficial to any mature patient with a traumatic brain injury. Congratulations to Dr. Khan and Dr. Hersh for securing funding to continue their life-altering research.”

Khan is the associate program director of the UConn School of Medicine’s Skeletal Biology and Regeneration Graduate Program and a member of the Laurencin Institute.
“This is a great example of the power of academic interdisciplinary medicine, where a talented surgeon brought a clinical problem to an engaged and creative scientist-engineer to work towards the betterment of patient care,” says Dr. Isaac Moss, chair of UConn Health’s Department of Orthopaedic Surgery. “When I connected Drs. Hersh and Khan five years ago, it was clear that these two faculty members would form a great partnership and it’s great to see fruits from this collaboration.”
Dr. Ketan Bulsara, chair of UConn Health’s Department of Neurosurgery, agrees.
“The interdepartmental collaboration between Dr. Hersh from neurosurgery and Dr. Khan from orthopedic surgery is just another example of our symbiotic clinical and research excellence that has the potential to transform patient care through our tripartite mission,” Bulsara says. “I congratulate them both on receiving this prestigious grant, and congratulate Dr. Jonathan Martin also for leading our exemplary pediatric neurosurgery team at Connecticut Children’s.”
Martin, a professor of surgery and pediatrics, directs Connecticut Children’s Division of Neurosurgery and holds its Paul M. Kanev Chair of Pediatric Neurosurgery.
“We have been privileged to partner with the UConn Health Department of Neurosurgery through the neurosurgery residency program, which has also expanded our access to new clinical and research partners,” Martin says. “The collaboration between Connecticut Children’s and UConn Health has accelerated the ability of exceptional faculty like Dr. Hersh to pursue answers to difficult questions that will benefit patients well beyond Connecticut and Western New England.”
The grant starts Feb. 1. While the research is in its very early stages, Khan says when the time comes, the work in the lab will be easily translatable.
“To me, this represents the best version of a clinician-research collaboration, where there is a clinical need looking for a solution, and there is a research solution looking for the ideal clinical application,” he says. “This demonstrates the power of and the need for clinician-scientist collaborations.”
The work was supported by the Assistant Secretary of Defense for Health Affairs endorsed by the Department of Defense, in the amount of $435,465.00, through the Peer Reviewed Medical Research Program under Award No. HT9425-25-1-0053. Opinions, interpretations, conclusions and recommendations are those of the author and are not necessarily endorsed by the Assistant Secretary of Defense for Health Affairs or the Department of Defense.
Latest UConn Today
- Sending off the Class of 2025The School of Dental Medicine celebrated the accomplishments of fourth-year students ahead of commencement
- After the Storm, a Rainbow for Cancer SurvivorsUConn Health Carole and Ray Neag Cancer Center Celebrates Survivors at the Yard Goats Game
- Podcast: Spray Away Severe DepressionDr. Caleb Battersby, UConn Health's director of interventional psychiatry, explains a treatment approach to severe depression that's providing hope
- Building Awareness of Ethical Animal ResearchOn April 17, UConn researchers took part in Biomedical Research Awareness Day.
- Congratulations Class of 2025Commencement is more than a ceremony—it’s a defining moment
- Neag School Class of 2025 Student Profile: Alexis Hastings“UConn had exactly what I was looking for and more.”